

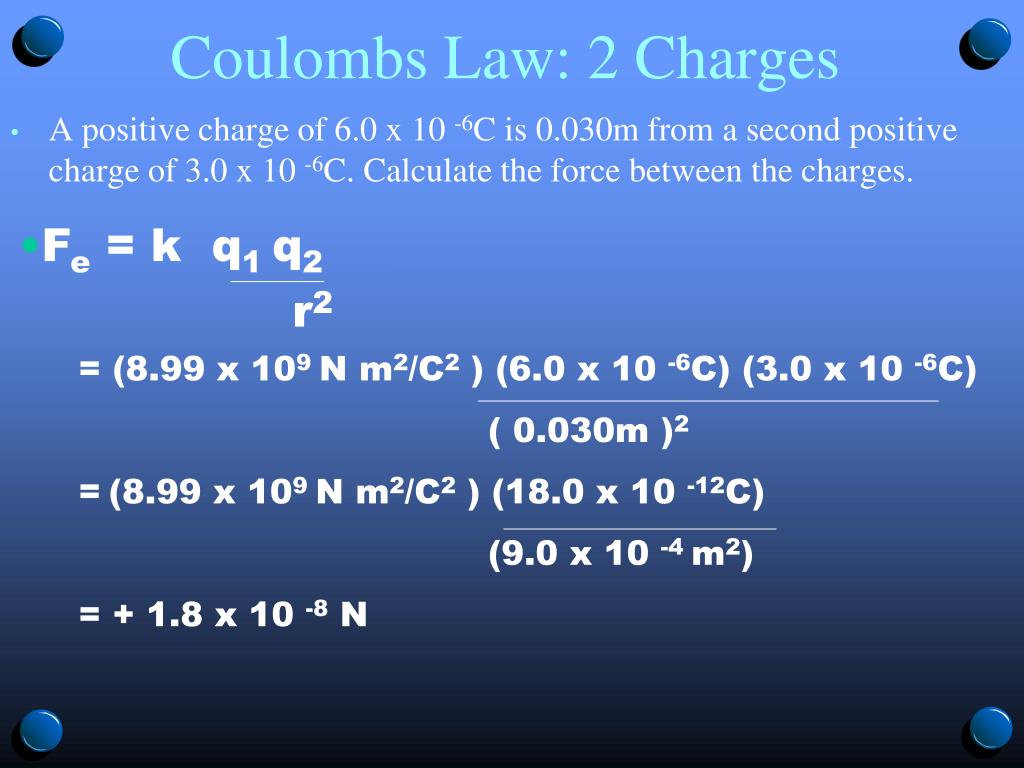
When the distance is tripled, the force is reduced to one-ninth.Ĭoulomb ’s law applies whether the two bodies in question have similar or opposite charges. When the distance between them is doubled, the force is reduced to one-fourth of its original value. Introducing a proportionality constant of k, Coulomb ’s law can be written as: q 1 × q 2 F = kr 2, What this law says is that the force between two charged bodies drops off rapidly as they are separated from each other. That is, the force between the two bodies is proportional to the product of their charges (q 1 × q 2 ) and inversely proportional to the square of the distance between them (1/r 2).

That mathematical expression was, indeed, comparable to the gravitation law. Coulomb ’s lawįrom this experiment, Coulomb was able to write a mathematical expression for the electrostatic force between two charged bodies carrying charges of q 1 and q 2 placed at a distance of r from each other. The amount of twist that develops in the fiber can be measured and can be used to calculate the force that produced the distortion. As they push away from each other, they cause the metal or silk fiber to twist. In this arrangement, a force of repulsion develops between the two adjacent balls. A third ball is then placed adjacent to the ball at one end of the horizontal rod and given a charge identical to those on the rod. Two small spheres are attached to opposite ends of the bar and given an electrical charge. The torsion balance consists of a non-conducting horizontal bar suspended by a thin fiber of metal or silk. The apparatus is known as a torsion balance. The French physicist designed an ingenious apparatus for measuring the relatively modest force that exists between two charged bodies. A decade later, however, two early English chemists, Joseph Priestley and Henry Cavendish, carried out experiments similar to those of Bernoulli and obtained qualitative support for a gravitation-like relationship for electrical charges.Ĭonclusive work on this subject was completed by Coulomb in 1785. Bernoulli ’s experiments were apparently among the earliest quantitative studies in the field of electricity, and they aroused little interest among other scientists. The first experiments in this field were conducted by the Swiss mathematician Daniel Bernoulli around 1760. Many assumed that such a law would follow the general lines of the gravitational law, namely that the force would vary directly with the magnitude of the charges and inversely as the distance between them. During the period 1760 – 1780, scientists began to search for a comparable law that would describe the force between two electrically charged bodies. Historyīy the early 1700s, Isaac Newton ’s law of gravitational force had been widely accepted by the scientific community, which realized the vast array of problems to which it could be applied. de Coulomb (1736 –1806), who formulated the law of electrical force that now carries his name. It was named after the French physicist Charles A. The internuclear distance in the gas phase is 175 pm.Īnswer: −3180 kJ/mol = −3.A coulomb (abbreviation: C) is the standard unit of charge in the metric system. Consequently, in accordance with Equation 4.1.1, much more energy is released when 1 mol of gaseous Li +F − ion pairs is formed (−891 kJ/mol) than when 1 mol of gaseous Na +Cl − ion pairs is formed (−589 kJ/mol).Ĭalculate the amount of energy released when 1 mol of gaseous MgO ion pairs is formed from the separated ions. \( \newcommand/mol\right )=-891\ kJ/mol \) īecause Li + and F − are smaller than Na + and Cl − (see Figure 3.2.7 ), the internuclear distance in LiF is shorter than in NaCl.


 0 kommentar(er)
0 kommentar(er)
